Right Atrial Tissue Morphology in Different Acquired Heart Diseases: A Pilot Study
Right Atrial Tissue Morphology in Different Acquired Heart Diseases: A Pilot Study
Abstract
Despite scientific progress, diseases of circulatory system are still the most common cause of mortality in Latvia and all Europe. Approximately 1–2 % of adult population in developed countries has heart failure, with the prevalence rising to ≥ 10 % among persons 70 years of age or older. One of the main principles of heart failure treatment is to target the underlying cause, for example, revascularisation and valve surgery. Understanding the pathophysiology and its clinical implications are important for the cardiovascular surgeon, because the therapeutic options may improve a patient’s outcome.
The aim of this study was to identify appearance and distribution of apoptosis, homeostasis regulating factors, innervations and ischemia markers in right atrial tissue in different acquired heart diseases.
In Pauls Stradiņš Clinical University Hospital during elective heart surgery were taken right atrial tissue fragments from 6 patients. The mean age was (mean ± SD) 70.3 ± 9.1 years (range 58–83 years) and there were 4 female patients. Tissues were processed for apoptosis, protein-gene peptide 9.5 (PGP 9.5), human atrial natriuretic peptide (hANUP), vascular endothelial growth factor (VEGF), chromogranin A and endothelin by means of biotin-streptavidin immunohistochemistry.
In all patients, a moderate to severe myocyte degeneration and vacuolisation was observed. In three patients, significant vascular sclerosis was found. The apoptotic index ranged from 24 % to 80 %. Mean number of TUNEL positive cardiomyocytes was significantly different among all specimens. All examined patients showed different expression of PGP 9.5. Numerous hANUP secreting cells were detected in all specimens, except patient No. 2, where this factor was detected in moderate cells. Few vascular endothelial growth factors were observed in endothelial cells in all specimens. Only in 2 patients, blood vessels contained endothelin positive endotheliocytes. Mostly this factor was seen in epicardium.
Different expression of PGP 9.5, hANUP, VEGF, chromogranin A and endothelin in right atrial tissue might characterise pathogenesis of different acquired heart diseases.
Large variation of apoptotic index seems to correlate to larger volume and longer untreated history of the affected disease in our pilot study patients in Latvia.
Introduction
Despite scientific progress, diseases of circulatory system are still the most common cause of mortality in Latvia and Europe [Nichols, 2012]. Approximately 1–2 % of adult population in developed countries has heart failure, with the prevalence rising to ≥ 10 % among persons 70 years of age or older [John, 2012]. One of the main principles of heart failure treatment is to address the underlying cause, for example, revascularisation and valve surgery. Understanding the pathomorphology and its clinical implications are important for the cardiovascular surgeon, because the therapeutic options may improve a patient’s outcome [Khoynezhad, 2004].
Programmed cell death (apoptosis) is a regulated mode of cell death in multicellular organisms [Hengartner, 1994]. Apoptosis of cardiac muscle cells has been identified as an essential process in the progression to heart failure [Empel, 2005]. Therefore, apoptosis could show heart failure before clinical symptoms have appeared.
Protein gene product 9.5 (PGP 9.5) is a specific protein used to visualise neuropeptide-containing innervations [Pilmane, 2011].
The heart secretes two natriuretic peptides, atrial (ANUP) and B-type (BNP). ANUP is secreted from atrial granules into the circulation in response to acute or chronic atrial stretch to physiologically act as antihypertensive and antihypervolaemic factor [Kuhn, 2012]. B-type natriuretic peptide is a cardiac neurohormone specifically secreted from the ventricles in response to volume expansion and pressure overload [Maisel, 2002]. The physiologic actions of BNP are similar to those of ANP and include reducing both cardiac preload and afterload by their natriuretic, diuretic, and vasodilatory actions [Nakagawa, 1995].
Vascular endothelial growth factors are major molecules controlling vascular growth and function, vascular homeostasis, permeability, and vasodilatation. They have been shown to be important for neovascularisation of the chronically ischemic adult heart [Karu, 2013].
Chromogranin A (ChgA) is a protein that is stored and released together with neurotransmitters and hormones in the nervous, endocrine and diffuse neuroendocrine systems [Glattard, 2006]. Cardiac ChgA is stored in atrial granules with cardiac natriuretic peptides. ChgA measurement has gained interest in cardiovascular disease, because increased plasma concentrations are associated with risk of clinical deterioration and death with acute coronary syndromes or chronic heart failure [Goetze, 2013]. ChgA levels have been found to reflect sympathetic activity, indicating that circulating ChgA levels may represent overall neuroendocrine activity. Increased activity in the sympathetic nervous system is a recognised risk factor for poor outcome in heart failure [Røsjø, 2010].
Endothelin is primarily released by endothelial cells, but it can also be synthesised and released by a variety of cell types, such as cardiac myocytes. Endothelins play an important role in cardiac and vascular pathology associated with heart failure [Zolk, 2000]. Endothelin is a potent vasoconstrictor and has inotropic, chemotactic and mitogenic properties. The overall action of endothelin is to increase blood pressure and vascular tone [Agapitov, 2002].
The aim of this study was to identify appearance and distribution of apoptosis, homeostasis regulating factors, innervations and ischemia markers in right atrial tissue in different acquired heart diseases.
Material and methods
In Pauls Stradiņš Clinical University Hospital’s Heart Surgery Centre during elective open heart surgery right atrial tissue fragments (~ 2 mm2) from 6 patients were taken. The material was obtained from the venous cannulae insertion site before cardioplegic solution was given. Tissue fixation was carried out immediately in the operating room and for this purpose already pre-prepared eppendorf tubes with saturated picric acid solution (2 % formaldehyde and 0.2 % picric acid in 0.1 M phosphate buffer (pH 7.2)) were used. Tissue fragments were transported to morphology laboratory, Institute of Anatomy and Anthropology at Rīga Stradiņš University. For 12 hours, tissue fragments were washed in 10 % sucrose phosphate buffer, embedded in paraffin and cut into 8 μm thick slices.
All tissue specimens were obtained in accordance with the ethical requirements of Rīga Stradiņš University.
Review pictures. Tissue for routine light-microscopical examination was stained with haematoxylin and eosin. To assess the cellular structure, all specimens were observed using Leica VM 6000B microscope.
Apoptosis. The cellular apoptosis was observed by terminal deoxynucleotidyl transferasemediated nick-end labelling method (TUNEL). All TUNEL positive cardiomyocytes in 3 randomly chosen non-overlapping fields of each specimen were counted. Also apoptotic index (the number of apoptotic cardiomyocytes as a percentage of all cardiomyocytes in one visual field) was determined.
Protein-gene peptide 9.5 (PGP 9.5). The slides were prepared to detect neuron specific proteinprotein- gene peptide 9.5 (439273A, working dilution 1 : 200, Invitrogen, USA).
Human atrial natriuretic peptide (hANUP). In order to assess the hormonal response, we determined hANUP (8515/6, working dilution 1 : 10, Dako, Denmark).
Vascular endothelial growth factor (VEGF). To identify chronic ischemia, we determined the presence of VEGF in blood vessels (SC7296, working dilution 1 : 50, Santa Crus Biotechnology, Inc., USA).
Chromogranin A. We also determined the neuroendocrine cell marker – chromogranin A (910216A, working dilution 1 : 100, Invitrogen, USA).
Endothelin. The slides were prepared also to detect endothelin releasing cells (ab2786, working dilution 1 : 250, Abcam, England).
Quantification of structures. For the quantification of structures, the semiquantitative counting method was used. The designations were as follows: 0 – negative reaction; 0 / + – occasionally positive structures in the view field; + – a few positive structures in the view field; + / ++ – few to moderate positive structures in the view field; ++ – moderate count of positive structures in the view field; ++ / +++ – moderate to numerous positive structures in the view field; +++ – numerous positive structures in the view field; ++++ – abundance of positive structures in the view field [Pilmane, 1998].
Patients. The mean age was (mean ± SD) 70.3 ± 9.1 years (range 58–83 years) and there were 4 female patients (Table 1). All patients were examined to the common procedure before heart surgery.
Table 1. Patient demographics
| No. | Age, years | Sex |
|---|---|---|
| 1 | 58 | Female |
| 2 | 62 | Male |
| 3 | 66 | Male |
| 4 | 73 | Female |
| 5 | 80 | Female |
| 6 | 83 | Female |
All patients had open-heart surgery (Table 2). 2 of them (No. 4, No. 6) had isolated aortic valve replacement because of aortic valve stenosis, 2 patients (No. 2 and No. 3) had coronary artery bypass grafting (CABG). One patient (No. 5) had concomitant CABG and mitral valve repair and one patient (No. 1) had reoperation – tricuspid valve replacement after mitral commissurotomy for rheumatic mitral valve stenosis in 1973 and mitral valve replacement, and tricuspid valve repair in 2000.
Table 2. Type of operation. Coronary artery bypass grafting (CABG)
| No. | Type of operation |
|---|---|
| 1 | “REDO” tricuspid valve replacement |
| 2 | CABG |
| 3 | CABG |
| 4 | Aortic valve replacement |
| 5 | CABG + mitral valve repair |
| 6 | Aortic valve replacement |
Echo and coronarography data are shown in Table 3 and Table 4. Almost all patients, except No. 5, had good left ventricular ejection fraction (LVEF) 55.2 ± 7.6 %. Patient No. 5 had a slightly (40 %) decreased ejection fraction. Two patients (No. 3 and No. 4) had moderate and severe pulmonary hypertension. All patients’ right atrial size was normal.
All patients, except No. 6, had anamnesis of coronary heart disease. Two of them (No. 1 and No. 4) had previous percutaneous transluminal coronary angioplasty (PTCA) and had no symptoms of coronary heart disease at the time of operation.
Table 3. Preoperative echo data
| No. | Echo | ||||||
|---|---|---|---|---|---|---|---|
| RAA, cm2 | LAVI, ml/m2 | RVSP, mmHg | LVEF, % | AR | MR | TR | |
| 1 | — | — | 55 | 55 | — | — | 4 |
| 2 | 17 | 35 | 22 | 58 | — | 1 | 1 |
| 3 | 15 | 32 | 30 | 58 | — | — | — |
| 4 | 13 | 33 | 30 | 65 | 2 | 1 | 1 |
| 5 | 17 | 46 × 67 mm | 66 | 40 | — | 4 | 2 |
| 6 | 17 | 37 × 52 mm | — | 55 | 1 | 1 | 1 |
RAA – right atrial area; LAVI – left atrial volume index, RVSP – right ventricular systolic pressure, LVEF – left ventricular ejection fraction, AR – aortic regurgitation, MR – mitral regurgitation, TR – tricuspid regurgitation.
Table 4. Preoperative coronarography data
| No. | Coronarography | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LM, % | LAD, % | LCX, % | RCA, % | |||||||
| prox | mid | dist | prox | mid | dist | prox | mid | dist | ||
| 1 | — | — | — | — | — | stent | — | — | — | — |
| 2 | — | — | 90 | 90 | — | 90 | 95 | — | 75 | 90 |
| 3 | — | 95 | 95 | — | — | — | — | — | 100 | — |
| 4 | — | stent | — | — | — | — | — | — | — | — |
| 5 | — | 75 | 100 | — | 100 | — | — | 100 | 100 | — |
| 6 | — | — | — | — | — | — | — | — | — | — |
LM – left main coronary artery, LAD – left anterior descending artery, LCX – left circumflex artery, RCA – right coronary artery.
Statistical analysis. All statistical analysis was performed with IBM SPSS Statistics 22. Statistical significance was considered at the level of p < 0.05. Data are given as mean ± standard deviation (SD).
Results
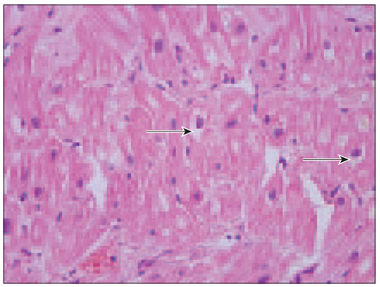
Review pictures. In all specimens, myocardial degeneration with diffuse vacuolation of cardiomyocytes was detected especially in perinuclear region (Figure 1).
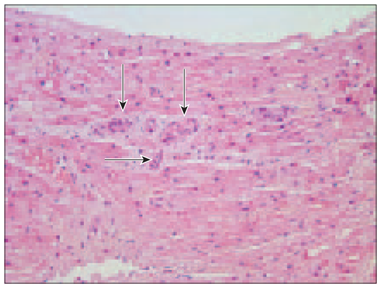
Cardiomyocytes and their nuclei in all specimens varied in size. In all patients, especially in Case No. 4, pyknotic nuclei were detected. In three patients (No. 2, No. 3, No. 6) significant vascular sclerosis was found (Figure 2), but in patient No. 5 endothelial cell proliferation was observed.
Figure 1. Vacuolar degeneration of cardiomyocytes (arrows, patient No. 4, H/Eo, × 400) | Figure 2. Almost completely sclerotised arterioles in myocardium (arrows, patient No. 3, H/Eo, × 200) |
|
|
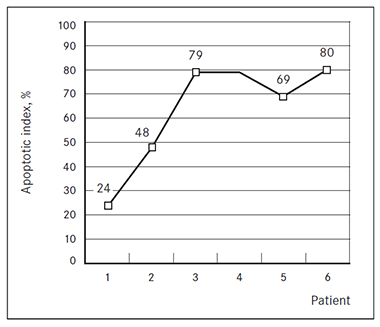
Apoptosis. The apoptotic index ranged from 24 to 80 % (Figure 3). The smallest (24 %) apoptotic index was determined in specimen No. 1.
Mean number of TUNEL positive cardiomyocytes (Table 5) was significantly different among all specimens (p = 0.000).
Figure 3. The apoptotic index of acquired heart disease affected right atrial tissue | Table 5. The number of apoptotic cardiomyocytes | |
| No. | The number of apoptotic cells, mean ± SD |
|---|---|---|
| 1 | 48.67 ± 14.19 | |
| 2 | 36.00 ± 15.00 | |
| 3 | 121.67 ± 26.50 | |
| 4 | 121.00 ± 15.87 | |
| 5 | 141.33 ± 8.33 | |
| 6 | 107.33 ± 14.74 | |
Protein-gene peptide 9.5. All examined specimens showed mainly numerous PGP 9.5 containing innervations (Table 6). However, an increased number of PGP 9.5 immunoactive innervations was observed in patient No. 4 (Figure 4), but in patient No. 5 PGP 9.5 was in few to moderate nerve fibres.
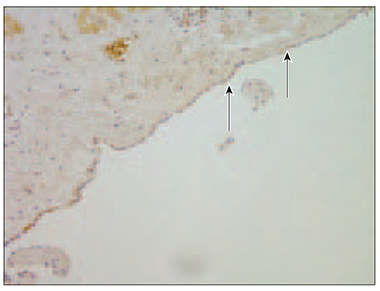
Interestingly, PGP 9.5 was found also in endothelium and in endocardial cells with quite good expression (Figure 5). Additionally, in patient No. 1, in scar tissue (after previous heart surgery, mitral valve replacement and tricuspid valve repair through right atriotomy), we found PGP 9.5 positive nerves only around the blood vessels.
Human atrial natriuretic peptide. Numerous hANUP secreting cells were detected in all specimens, except patient No. 2, where this factor was detected in moderate cells (Table 6).
Figure 4. Protein-gene peptide 9.5 positive nerve fibres among connective tissue in myocardium (arrows, patient No. 4, IMH, × 200) | Figure 5. Rich PGP 9.5 expression in endocardial layer (arrow, patient No. 5, IMH, × 400) |
|
|
Table 6. Immunohistochemical analysis
| No. | PGP 9.5 | hANUP | VEGF | Chromogranin A | Endothelin | |
|---|---|---|---|---|---|---|
| Endothelium | Endocardium | |||||
| 1 | + / ++ | +++ | + | 0 | ++ | + |
| 2 | +++ / ++++ | +++ | + | +++ | + | + |
| 3 | +++ | +++ | + | + | 0 | 0 / + |
| 4 | +++ | ++ | + | ++ | ++ | ++ |
| 5 | +++ | +++ | + | + | ++ / +++ | ++ |
| 6 | +++ | +++ | + | 0 | 0 / + | 0 / + |
PGP – protein-gene peptide 9.5. hANUP – human atrial natriuretic peptide. VEGF – vascular endothelial growth factor.
The designations were as follows: 0 – negative reaction; 0 / + – occasionally positive structures in the view field; + – a few positive structures in the view field; + / ++ – few to moderate positive structures in the view field; ++ – moderate count of positive structures in the view field; ++ / +++ – moderate to numerous positive structures in the view field; +++ – numerous positive structures in the view field; ++++ – abundance of positive structures in the view field.
Vascular endothelial growth factor. Few vascular endothelial growth factors were observed in endothelial cells in all specimens (Table 6). In patient No. 4 VEGF mostly was found in endocardium (Figure 6).
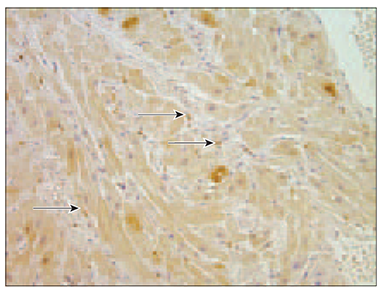
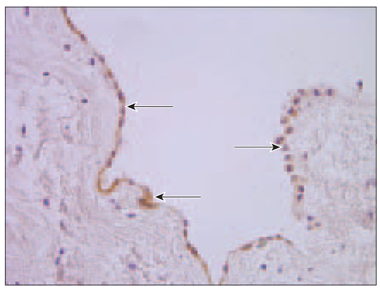
Chromogranin A. There were some blood vessels, which few endothelial cells contained chromogranin, in patient No. 5 (Table 6). In specimens No. 2 and No. 4 were no signs of chromogranin in blood vessels at all, but few positive cells were found in the endocardium. In patient No. 6 no chromogranin was observed. There were no chromogranin containing blood vessels in specimen No. 3., but factor positive cells particularly were pronounced in epicardium (Figure 7).
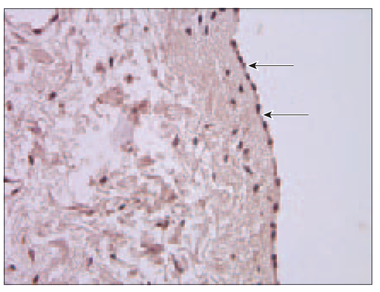
Endothelin. Only in 2 patients (No. 5 and No. 4) blood vessels contained endothelin positive endotheliocytes (Table 6). Mostly this factor was seen in epicardium (Figure 8).
Figure 6. Moderate nucleus of VEGF immunoreactive cells in endocardial layer (arrows, patient No. 4, IMH, × 200) | Figure 7. Chromogranin A containing epicardium (arrows, patient No. 3, IMH, × 400) |
|
|
Figure 8. Endothelin in epicardium (arrows, patient No. 4, IMH, × 400) | |
|
Discussion
Review Pictures. In all patients, a moderate to severe myocyte degeneration and vacuolisation was observed, and it is a marker of heart failure [Ahmed, 2010]. Besides, preoperatively performed echo showed good or only slightly reduced left ventricular ejection fraction. It seems that these patients have a latent heart failure, without the true left ventricular ejection fraction decline yet.
Apoptosis. Cardiomyocyte apoptosis has been associated with the pathogenesis of heart failure [Empel, 2005]. There were statistically significant differences in apoptotic index and apoptotic cell count in right atrial tissue among all patients in our study, but no relationships between reduced left ventricular ejection fraction or clinical heart failure classes and apoptosis were observed. It is noteworthy that the lowest apoptotic index was found in youngest patients (age 58 and 62 years/apoptotic index 24 % and 48 %).
It is interesting that apoptotic index in our patients was very high (range from 24 to 80 %) if we compare to other studies. So, Narula (1996) determined apoptotic index in 7 explanted hearts from patients undergoing heart transplantation at Massachusetts General Hospital and it ranged from 5 to 35.5 % [Narula, 1996]. Thus, we speculate on the differences between these patient groups, predicting that probably our patients received surgical treatment later than American patients.
Protein-gene peptide 9.5. Protein-gene peptide 9.5 is a specific marker of nervous tissue [Chow, 2001]. Relatively good innervations in all specimens were observed with one exception – patient No. 5, proving probably “weak heart syndrome” in relation to all other patients. On the other hand, proteingene peptide was particularly pronounced in patient No. 4. It was the patient, who underwent aortic valve replacement because of aortic valve stenosis due to calcific degeneration, suggesting that more pronounced remodeling of heart muscle compensatory connects to the increase of neuropeptide containing innervations.
Human atrial natriuretic peptide. Atrial myocardiocytes contain neuroendocrine granules and these granules secrete atrial natriuretic hormone when the atria are stretched excessively. Numerous hANUP secreting cells in all specimens were detected, except patient No. 2, where such cells were only slightly fewer. Atrial natriuretic peptide is involved in the maintenance of arterial blood pressure and intravascular volume homeostasis [Kuhn, 2012]; it concludes that there is active hormonal response to ensure homeostasis in all cases reviewed, except the patient No. 2.
Vascular endothelial growth factor. Vascular endothelial growth factor (VEGF) is an angiogenic protein that can stimulate collateral vessel development in the ischemic myocardium [Pearlman, 1995]. Karu et al. (2013) demonstrated that patients with stable coronary artery disease have significantly lower serum levels of vascular growth factors compared with data of healthy volunteers [Karu, 2013]. In this study, no relationship between coronary heart disease and expression of vascular endothelial growth factor in right atrial tissue has been observed. Additionally, the presence of only few VEGF containing cells in our patients indicates the lack of serious tissue ischemia. The increase of VEGF in some patient endocardial layer raises the question about the involvement of other heart structures except myocardial blood vessels in the tissue suffering from the heart disease and request further research.
Chromogranin A. Heart failure is a syndrome comprising cardiac dysfunction and neurohumoral activation [Goetze, 2014]. In 2007, Pieroni et al. for the first time demonstrated that human ventricular myocardium produces and releases chromogranin A [Pieroni, 2007]. This study allowed to determine chromogranin A in right atrial tissue and it was also found in endothelium (patient No. 5), endocardium (patient No. 2 and No. 4) and epicardium (patient No. 3). That means that probably these heart structures can change a phenotype and produce neuroendocrine prohormones important for the diseased tissue functions, from the one side, and, from the other side, stimulate remodeling of heart tissue [Pieroni, 2007].
Endothelin. Increased production and biological activity of the potent vasoconstrictor and pro-inflammatory peptide endothelin is a hallmark of endothelial dysfunction [Böhm, 2007], while the endothelial dysfunction is associated with accelerated progression of heart failure [Fischer, 2005]. In the retrospective study, most of all endothelin was found in patients No. 4 and No. 5 and one of them had the poorest left ventricular ejection fraction – 40 % and the highest pulmonary hypertension (RVSP 66 mmHg). The role of endothelin end endothelial dysfunction in pulmonary hypertension development has been demonstrated in several studies [Galie, 2004].
Conclusions
Different expression of PGP 9.5, hANUP, VEGF, chromogranin A and endothelin in right atrial tissue might characterise pathogenesis of different acquired heart diseases.
Large variation of apoptotic index seems to correlate to a larger volume and longer untreated history of affected disease in our pilot study patients in Latvia.
References
- Agapitov A. V., Haynes W. G. Role of endothelin in cardiovascular disease // Journal of Renin-Angiotensin-Aldosterone System, 2002; 3 (1): 1–15.
- Ahmed M. I., Gladden J. D., Litovsky S. H., et al. increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction > 60 % // Journal of the American College of Cardiology, 2010; 55 (7): 671–679.
- Böhm F., Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease // Cardiovascular Research, 2007; 76: 8–18.
- Chow L. T. C., Chow S. S. M., Anderson R. H., Gosling J. A. Autonomic innervations of the human cardiac conduction system: Changes from infancy to senility. An immunohistochemical and histochemical analysis // The Anatomical Record, 2001; 264: 169–182.
- Empel V. P. M., Bertrand A. T. A., Hofstra L., et al. Myocyte apoptosis in heart failure // Cardiovascular Research, 2005; 67: 21–29.
- Fischer D., Rossa S., Landmesser U., et al. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death // European Heart Journal, 2005; 26 (1): 65–69.
- Galie N., Manes A., Branzi A. The endothelin system in pulmonary arterial hypertension // Cardiovascular Research, 2004; 61 (2): 227–237.
- Glattard E., Angelone T., Strub J. M., et al. Characterisation of natural vasostatin-containing peptides in rat heart // The FEBS Journal, 2006; 273: 3311–3321.
- Goetze J. P., Alehagen U., Flyvbjerg A., Rehfeld J. F. Making sense of chromogranin A in heart disease // The Lancet Diabetes & Endocrinology, 2013; 1 (1): 7–8.
- Goetze J. P., Hilsted L. M., Rehfeldand J. F., Alehagen U. Plasma chromogranin A is a marker of death in elderly patients presenting with symptoms of heart failure // Endocrine Connections, 2014; 3: 47–56.
- Hengartner M. O., Horvitz H. R. Programmed cell death in Caenorhabditis elegans // Current Opinion in Genetics and Development, 1994; 4: 581–586.
- John J. V., Adamopoulus S., Anker S. D., et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012 // European Heart Journal, 2012; 33: 1787–1847.
- Karu I., Starkopf J., Zilmer K., Zilmer M. Growth factors serum levels in coronary artery disease patients scheduled for bypass surgery: Perioperative dynamics and comparisons with healthy volunteers // http://dx.doi.org/10.1155/2013/985404 (accessed on 10.06.2014).
- Khoynezhad A., Jalali Z., Tortolani A. J. Apoptosis: Pathophysiology and therapeutic implications for the cardiac surgeon // The Annals of Thoracic Surgery, 2004; 78 (3): 1109–1118.
- Kuhn M. Endothelial actions of atrial and B-type natriuretic peptides // British Journal of Pharmacology, 2012; 166 (2): 522–531.
- Maisel A., Krishnaswamy P., Nowak R. M., et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure // The New England Journal of Medicine, 2002; 347 (3): 161–167.
- Nakagawa O., Ogawa Y., Itoh H., et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy // The Journal of Clinical Investigation, 1995; 96: 1280–1287.
- Narula J., Haider N., Virmani R., et al. Apoptosis in myocytes in end-stage heart failure // The New England Journal of Medicine, 1996; 335 (16): 1182–1189.
- Nichols M., Townsend N., Scarborough P., Rayner M. European Cardiovascular Disease Statistics. 2012 edition // http://www.escardio.org/about/documents/eu-cardiovascular-disease-statistics-2012.pdf (accessed on 10.06.2014).
- Pearlman J. D., Hibberd M. G., Chuang M. L., et al. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis // Nature Medicine, 1995; 1 (10): 1085–1089.
- Pieroni M., Corti A., Tota B., et al. Myocardial production of chromogranin A in human heart: A new regulatory peptide of cardiac function // European Heart Journal, 2007; 28: 1117–1127.
- Pilmane M., Ozolina L., Abola Z., et al. Growth factors, their receptors, neuropeptide-containing innervation, and matrix metalloproteinases in the proximal and distal ends of the esophagus in children with esophageal atresia // Medicina, 2011; 47 (8): 453–460.
- Pilmane M., Rumba I., Sundler F., Luts A. Patterns of distribution and occurrence of neuroendocrine elements in lungs of humans with chronic lung diseases // The Journal of Latvian Academy of Sciences, 1998; 52: 144–152.
- Røsjø H., Masson S., Latini R., et al. Prognostic value of chromogranin A in chronic heart failure: Data from the GISSI-Heart Failure trial // European Journal of Heart Failure, 2010; 12: 549–556.
- Zolk O., Böhm M. The role of the cardiac endothelin system in heart failure. // Nephrology Dialysis Transplantation, 2000; 15 (6): 758–760.